The first malaria treatment for infants and very young children has been approved for use.
It is expected to be launched in African countries within weeks.
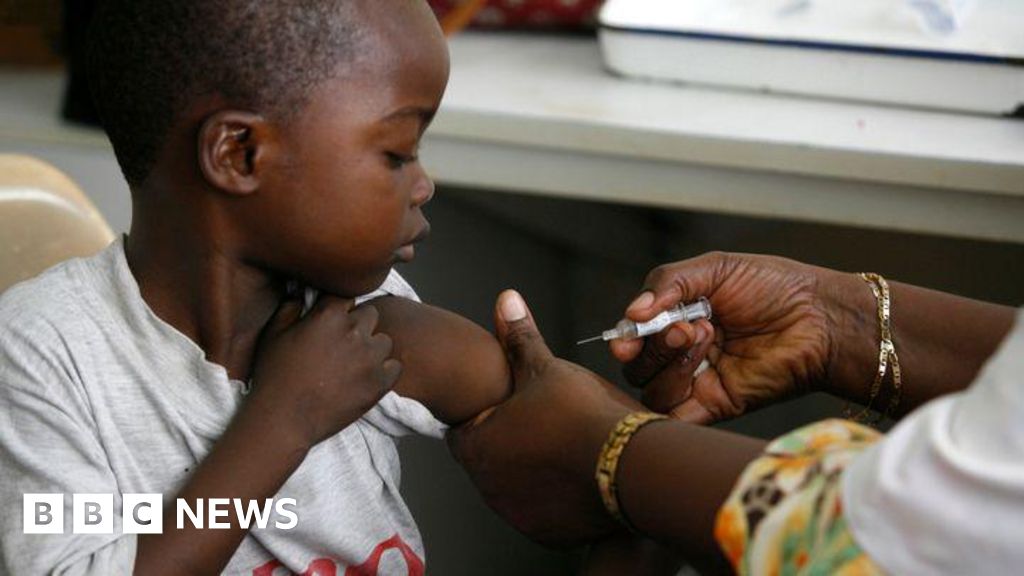
So far, there are no malaria drugs specifically targeting babies.
Instead, they have accepted versions designed for older children, which are at risk of overdose.
In 2023 – the year of latest data available – malaria is linked to the deaths of about 597,000 people.
Almost all deaths are in Africa, with about three-quarters of them being children under the age of five.
There are indeed malaria treatments for children, but until now, the smallest babies and children weigh less than 4.5 kg or 10 pounds.
Instead, they have been treated with medications designed for older children.
However, this poses risks, as these older children’s doses may still be the baby’s liver function and their body handles it differently.
Experts say this leads to the so-called “treatment gap.”
Now, a new medicine developed by Novartis Pharmaceuticals has been approved by Swiss authorities and is likely to be launched in regions and countries with the highest malaria rates in a few weeks.
Novartis plans to introduce it to a largely nonprofit.
This is a big moment, said the company’s CEO Vas Narasimhan.
“For the past three decades, we have maintained the curriculum in the battle against malaria, working tirelessly to provide the scientific breakthroughs most needed.
“Together with our partners, we are honored to further develop the first clinically proven malaria treatment for newborns and young babies, ensuring that the smallest and most vulnerable individuals can eventually receive the care they deserve.”
The drug, known in some countries as Coartem Baby or Riamet Baby, was developed by Novartis in partnership with Malaria Venture Capital (MMV), a Switzerland-based nonprofit that was originally backed by the governments of the UK, Switzerland and the Netherlands, as well as the World Bank and the Rockefeller Foundation.
Eight African countries have also participated in the evaluation and trial of the drug, which is expected to be the first to obtain the drug.
MMV CEO Martin Fitchet said this is another important step toward ending the huge casualties caused by malaria.
“Malaria is one of the deadliest diseases in the world, especially in children. But with the right resources and focus, it can be eliminated.
“Coartem Baby’s approval provides the necessary medications and has optimized doses to treat originally neglected patients and provides valuable supplements to the antimalarial toolbox.”
Dr. Marvelle Brown, associate professor at the University of Hertfordshire School of Health, Medicine and Life Sciences, said this should be seen as a major breakthrough in saving babies and young children’s lives.
“The mortality rate of malaria infection, especially in sub-Saharan Africa, is very high – more than 76% of deaths occur in children under the age of five.
“The increased death to malaria in infants with sickle cell disease is mainly due to a weak immune system.
“From a public health perspective, Novartis’s manufacturing of such nonprofits can help reduce inequality in access to health care.”